The Difference Between Heat and Temperature


In my last blog post, I wrote about heat pumps. I also discussed the difference between heat and temperature. Unfortunately, there’s the potential to confuse things linguistically here. We’re talking about something being hot or cold. We’re already using the word hot. And when the winter in northern Hesse gets really cold, people often say they “want to go somewhere hot,” and fly away on holiday.
Yet caution is required at this point, as what we colloquially refer to as “heat” is, in technical and scientific terms, completely wrong. When we talk colloquially of heat, in most cases, we actually mean temperature.
Let’s take the example of a burning match, which gets quite hot, i.e. it has a high temperature. It goes without saying that you can’t “heat” a whole house with it though: to do that you would certainly need a lot of matches! We therefore talk of the amount of heat.
Heat is a form of energy, and can be generated from gas, electricity or oil. In this respect, the good thing is that energy can be transformed from one form to another. We saw this in the last example: electrical energy (electricity) heats the hotplate, which heats the water in the saucepan.
Heat is therefore a form of energy, and the temperature describes the state of a medium (air, water).
Transferring energy can only take place from a higher state to a lower state: in the case of heat, from hot to cold; in the case of pressure, from the high pressure in the bicycle pump to the lower pressure in the tyre; when electrically charging a battery, from a high to a low voltage. In terms of heat, we also talk of the second principle of thermodynamics.
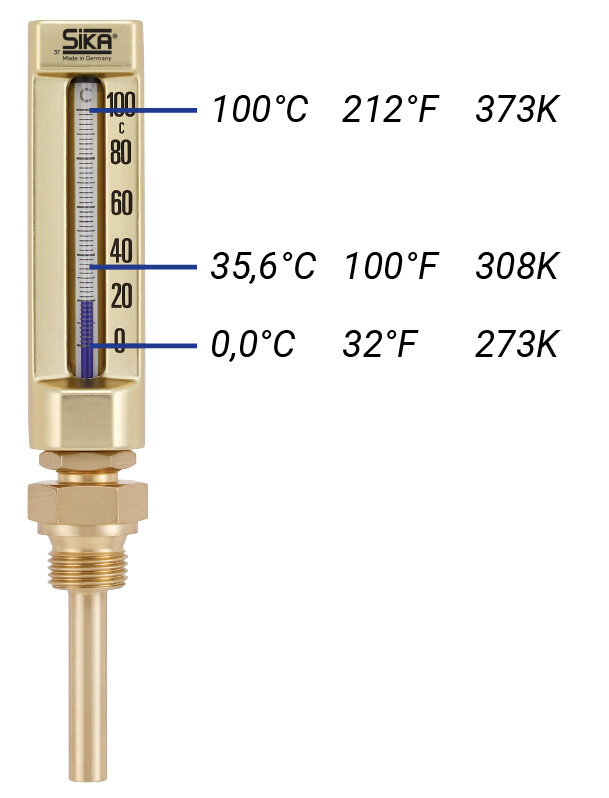
Here in Germany, temperature is generally measured in degrees Celsius (°C). This goes back to the Swedish physicist Anders Celsius, who fixed the freezing point of water at zero and the boiling point at 100. In between, there is a scale which is divided into 100 equal steps (grading = dividing into steps).
In other countries (the USA), Fahrenheit (°F) is also frequently used. This unit was developed by the German physicist Daniel Gabriel Fahrenheit. On a cold winter’s day in Gdansk, Fahrenheit succeeded in freezing a salt-water solution at the lowest possible temperature. He placed the temperature of the human body at the top of the scale which he set to 100. At that time, it wasn’t yet possible to measure the temperature of the human body so accurately, so the 100-degree point of the Fahrenheit scale stops at 35.6°C instead of 37°C. As the Fahrenheit scale is also divided into degrees, we talk of degrees Fahrenheit.
Incidentally, Mr. Fahrenheit also did a favour for SIKA. He is regarded as the inventor of the mercury thermometer and therefore the forerunner of our mercury-free industrial thermometers of today. The SIKA thermometers are also available with the Fahrenheit scale.
Conversion formulae:
(°C x 1.8) + 32 = °F
°C + 273 = K
Other units of measurement for temperature also exist (such as the Réaumur which was once used in France). The universally-valid Kelvin (K) unit is very important, however. It is named after the British scientist William Thomson, Lord Kelvin. This unit is not graded either, and also works in temperature ranges which do not bear any relation to water.
Kelvin is not graded, i.e. it is simply Kelvin, not degrees Kelvin. From the technical perspective, temperature differences are also indicated in Kelvin and not in °C.
Kelvin is one of 7 base units of the international system of units (SI). It is universally valid, and its absolute zero point, if measured in degrees Celsius, is -273.15°C. It doesn’t get colder than this anywhere in the universe.
SI units
| Base quantity | Symbol/name | Name of the measurement unit | Unit symbol |
|---|---|---|---|
| Time | t | Second | s |
| Length | l | Meter | m |
| Mass | m | Kilogram | kg |
| Electric current | I | Ampere | A |
| Thermodynamic | T | Kelvin | K |
| Amount of substance | n | Mol | mol |
| Luminous intensity | Iv | Candela | cd |